2025 UPDATE Spreading Varroa Resistant Traits
- Posted
2025 Update
Dr Melissa Oddie has done some outstanding recent research worthy of mention in the last couple years on this topic. Below are the links to two resources for your review.
- Reproductive success of the parasitic mite (Varroa destructor) is lower in honeybee colonies that target infested cells with recappingSteve Riley (Westerham Beekeepers)
- Resisting Varroa – Dr Melissa Oddie – Youtube
Background
The first discovery of Varroa jacobsoni (later renamed Varroa destructor) in the United States was made on Sept. 25, 1987, in Florida on colonies owned by a Wisconsin beekeeper. This beekeeper, Gary Oreskovic, was a supplier of package bees that were combined with packages from other beekeepers and sold to companies and individuals in Wisconsin. The initial infested hives were depopulated (killed). Yet by October 20 of that same year 19 of Florida’s 67 counties had positive finds for the mites, and within two years the mites were found in 19 states within the United States. They are now found in every part of the Americas where bees are kept. Cornell Prof. Roger A. Morse reported that the source of the queens that introduced the mites into North America was somewhere in South America, imported illegally by a commercial beekeeper.
Because European beekeepers had experienced the wave of varroa across that continent, there were pre-existing chemical control methods in production that were easily adapted within the United States. A compound called Apistan (fluvalinate) was impregnated into wooden strips in initial treatments in Florida and elsewhere. These were replaced by the availability of plastic Apistan strips that were widely sold to control mite numbers. There was little discussion about ‘should we treat’, but rather the driving insistence that we develop treatment methods that were cost effective and relatively inexpensive. This did not stop the wide-scale use of home-made delivery methods to administer fluvalinate (tau-fluvalinate is sold as Mavrik and Klartan for insecticidal and acaracidal action on aphids, trips, leafhoppers, whitefly, beetles and spider mites). Fluvalinate is a pyrethroid that acts on the insect nervous system. Because it was available for purchase by agricultural producers, many beekeepers developed their own control methods using the agricultural preparation of the compound. In doing so they both over- and under-dosed the colonies where they were attempting to achieve mite control. Over-dosing provided evidence of toxic effects to queens, drones, workers and lead to widespread comb contamination. Where lower than recommended levels were used, there were a larger number of mites that survived the treatment, ultimately leading to mite resistance to the compound. Because of the wide-spread resistance, the compound is now used only when in rotation with other mite control molecules.
Untreated colonies suffered horribly as the mites swept across the country. Beekeepers reported the deformity of worker bees at the time they should emerge from their cells, and the appearance of damaged wings as a result of the mites feeding on the bees. The eventual outcome was the death of the colony. Within a few years there were reports of colonies that were still alive after the mites had destroyed the rest of the colonies in the apiary. Feral bee colonies died from the mites as well, and gardeners and naturalists quickly noticed the lack of honeybees on vegetable and flower gardens, as well as a decline in the natural food bees pollinate for birds and wildlife. With the absence of competition from honeybee foragers for the same food supply, many naturalists and scientists noticed an increase in the number of bumble bees and other native pollinators. It was an interesting exercise to observe the change in the ecosystem as honeybees were somewhat suddenly removed.
The survival colonies were of interest to many beekeepers, and many small-scale producers used these few remaining colonies as the basis of their slow rebuild of bee colony numbers. The progress was slow. Researchers noticed too and sent out a call for beekeepers to ship them their queens and they started a stock of survivor queens. These programs developed the first Suppressed Mite Reproduction or SMR strain of honeybees. Later it was shown that the SMR trait is a form of hygienic behavior, and the SMR trait was renamed the Varroa Sensitive Hygienic or VSH trait. The mechanism explaining the SMR trait has not been described, but it was found that bees with the SMR trait were very hygienic and were able to remove varroa-infested pupae from capped brood cells. They also suggested that SMR bees may selectively remove pupae having reproductive mites. It was shown that the brood cells with reproductive mites were opened and removed by the bees with the hygienic trait, while the single foundress mite that is nonproductive was not removed and was assumed to emerge from the worker cell when the worker bee emerged. Because she had not produced offspring, she was not removed from the cell by hygienic bees.
Introduction
The near-globally distributed ecto-parasitic mite of the Apis mellifera honeybee, Varroa destructor, has formed a lethal association with Deformed wing virus, a once rare and benign RNA virus. In concert, the two have killed millions of wild and managed colonies, particularly across the Northern Hemisphere, forcing the need for regular acaricide application to ensure colony survival. However, despite the short association (in evolutionary terms), a small but increasing number of A. mellifera populations across the globe have been surviving many years without any mite control methods. This long-term survival, or Varroa resistance, is consistently associated with the same suite of traits (recapping, brood removal and reduced mite reproduction) irrespective of location.
Mite-resistance studies have found in these colonies high rates of recapping of infested worker cells (77%), high removal of mites (80%) and corresponding low mite fertility (r = 0.77). These are all traits found in all naturally evolved Varroa-resistant populations. It’s even been confirmed that in Cuba there is the world’s largest European mite-resistant population with 220,000 colonies that have been treatment-free for over two decades, illustrating the power of natural selection. Cuban honeybees are also highly productive, 40–70 kg of honey produced annually, and are mild mannered. Cuba is an excellent example of what is possible when honeybees are allowed to adapt naturally to Varroa with minimal human interference.
Resistance and Population
Studies suggest that resistance is a sequence of events that generate the key traits (increased recapping, brood removal and mite infertility) rather than a single trait. Also, this enhanced expression of these three key traits is common among resistant populations. This independent occurrence of the key traits within colonies across the world could be an example of parallel evolution, because while the recapping and removal behaviors predate Varroa, they have been co-opted to control Varroa, recapping is a rare trait in susceptible (mite-naïve) colonies but occurs at low and high levels in susceptible and resistant colonies respectively. Similarly, other traits such as brood suppression of mite reproduction, or DWV tolerance may be complementary. There is also likely to be a mite element to resistance which could be illuminated by further studies into the coevolution of A. mellifera and Varroa. As resistance is an overall honeybee population level trait rather than a single colony trait, a resistant colony becomes vulnerable if moved out of its local population and could collapse if a sudden influx of mites occurs due to excessive (40–60%) brood removal. This may explain why resistant colonies moved out of their population typically do not survive. This raises questions about the impact of migratory practices and bee sales on the ability of resistant colonies to survive.
Resistance and Queens
The mite levels in colonies headed by mite-naïve queens or their daughters significantly exceed that found in colonies headed by resistant queens or their daughters. Evidence shows that V. destructor levels on the adult bees and in the worker, brood increased significantly in colonies headed by mite-naïve queens or their daughters. In contrast, mite levels remained constant in colonies headed by a resistant queen or their daughters. Therefore, to propagate mite-resistant traits, beekeepers only need to re-queen a colony with a locally mated queen from an established resistant population. Also, queens raised from this stock can be used to re-queen susceptible colonies to allow beekeepers to manage their bees without the need for miticide treatments.
Evolution
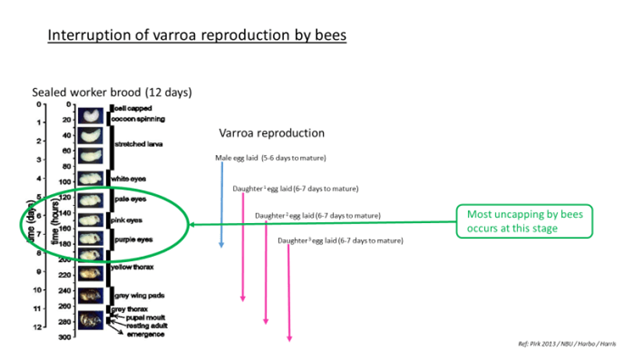
Some isolated A. mellifera populations are known to survive infestations by means of natural selection, largely by suppressing mite reproduction, but the underlying mechanisms of this are poorly understood. It’s assumed that a cost-effective social immunity mechanism has evolved rapidly and independently in naturally V. destructor-surviving A. mellifera populations. Worker bees of populations uncapped/recapped worker brood cells more frequently and targeted mite-infested cells more effectively than workers in local susceptible colonies. Experiments confirmed the ability of uncapping/recapping to reduce mite reproductive success without sacrificing nestmates. These results provide striking evidence that honeybees can overcome exotic parasites with simple qualitative and quantitative adaptive shifts in behavior. Due to rapid, parallel evolution in honeybee populations this appears to be a key mechanism explaining survival of mite infested colonies.
Social Immunity
Eusocial insect colonies (complex social structures of individuals within a colony) can be regarded as superorganisms in which cooperating individuals of overlapping generations are analogous to cells in a multicellular organism. Such cooperation includes reproductive division of labor, brood care and parasite defense via social immunity. Social immunity involves those behavioral, physiological or organizational traits that enhance colony health, irrespective of their consequences for the health of individual bees.
In respect to Varroa resistant traits, there is clear evidence that recapping is a cost-effective social immunity mechanism that helps, in part, explain the natural survival of European honeybees with unmanaged V. destructor infestations. Removal of infested brood and other factors may have also contributed to the natural mite-survival of these populations. Indeed, most likely the phenotype of naturally V. destructor-surviving honeybee colonies is determined by a series of host traits, local genotype-environment interactions, pathogen variation, resource availability and beekeeping management. However, due to its rapid and parallel evolution in surviving populations and the significant impact on mite reproductive success, recapping appears to be a common and previously overlooked key mechanism for colony survival. Brood removal, though potentially linked to recapping is unlikely to be a primary mechanism since the loss of individuals would compromise colony competitiveness.
Uncapping/Recapping
Studies have found that uncapping/recapping by adult bees is a behavioral mechanism mitigating mite infestations. Mite offspring mortality has also been found to be higher in recapped cells further supporting recapping as an effective mechanism promoting honeybee resistance and survivability. However, with the knowledge that bees are selective in their targeting of infested cells; if uncapping workers focus their efforts on cells with the most mite reproduction and ignore cells with a lower reproduction, the observed rates of reproduction in recapped and untouched cells may become similar, but through different mechanisms. Recapped cells would have a lower mite reproductive rate due to the direct effect of recapping, while cells with naturally low or non-reproductive mites would be ignored by uncapping bees, and consequently be underrepresented in the recapped cohort. Therefore, the absence of differences in mite reproductive success between recapped and untouched cells is uninformative due to the selection bias introduced by the bees. However, experimental uncapping of brood cells showed that recapping can reduce mite reproductive success directly, without sacrificing nestmates. The efficacy of recapping as a natural mechanism for reducing mite reproductive success could only be shown by combining the survey data with the experimental uncapping, since the latter excluded any biased targeting of mite-infested cells. Experiments have demonstrated that recapping behavior can cause reduced colony-level mite reproductive success and is not only correlated with it. The data therefore provide clear evidence that recapping is a cost-effective social immunity mechanism that helps, in part, explain the natural survival of European honeybees with unmanaged V. destructor infestations and appears to be an integral part of brood care and pathogen defense.
Pupae Removal and Deformed Wing Virus (DWV)
While survivor colonies in Brazil and South Africa are both resistant to varroa mites, they express differences in their DWV genotypes. However, in both countries, the viral load is significantly lower than in the UK or the USA. This may be explained by the finding that, in both Brazil and South Africa, the adult workers detect and remove a greater amount of infested brood than is the case in the Northern Hemisphere, thereby reducing the viral burden on the colony. Although the highest viral loads were detected in mite-infested pupae, over 50% are removed by the bees prior to maturity, and the remaining infested pupae have reduced longevity as adults. Both of these factors greatly reduce the viral load in adult bees, as was seen in South Africa and Brazil, but not in the UK or the USA, where the rate of removal of mite-infested pupae is low.
Regarding the cannibalization of DWV-infected pupae, it’s been shown that this behavior results in higher levels of DMV virus in worker bees and that the acquired virus was then transmitted between bees via trophallaxis (exchange of regurgitated liquid food between individuals), allowing circulation of Varroa-vectored DWV variants without the mites. Despite the known benefits of hygienic behavior, it is possible that higher levels of VSH activity may result in increased transmission of DWV via cannibalism and trophallaxis but will normally level out to an acceptable infection rate as mite counts are also reduced.
Treatments
As a comparative baseline, 2,872 beekeeper responses were gathered from an estimated 30,000 UK beekeepers belonging to 242 bee-associations in the winter of 2020/21. The survey indicated that the majority (72-79%) of UK beekeepers are still treating their bees for Varroa, typically twice-yearly using chemical-based methods. Six percent or 1,800 UK beekeepers were treatment-free for six years or more. This is reflected by the finding that 78 associations out of 242 consist of responders who entirely treated, while only four associations had more than 75% of their members that were non-treating.
Nonetheless, the majority (72–79%) of UK beekeepers are still treating their colonies to control Varroa numbers. Beekeepers in the UK are predominantly using a single or bi-annual chemical treatment regime. The order of popularity starting with the most common is oxalic acid, thymol, amitraz and formic acid. Only 20% of beekeepers in this study were adopting a combination of methods approach.
The success of any treatment method or treatment-free beekeeping is determined by their overwinter losses. Over the last 14 years in the UK over-wintering annual colony losses have ranged from over 30% to 8% with an average of 18%. Principal causes of colony losses reported by beekeepers in 2021 were queen-related problems (24%), isolation starvation (21%), weather-related 17%, and Varroa was just 4% reflecting the situation that the mite is no longer considered a major problem by beekeepers currently in the UK. However, Varroa could be contributing to some of the other colony losses indirectly.
One of the dangers of switching over to an intervention free-treatment regime involves a period of high winter losses whilst the bees develop resistance. This is due to re-invasion from collapsing colonies to surviving surrounding colonies or by visits from infested non-natal bees. Several strategies have been adopted at both local and national level to encourage mite-resistant honeybees. Catching free-living swarms from locations where they appear to have persisted for many years has been a successful strategy in the UK and Hawaii. Others have reduced the frequency of mite-treatments by selectively treating colonies with high mite levels, or to use biomechanical methods.
Already many countries (South Africa, Brazil, Mexico, Cuba etc.), along with beekeepers in the UK and elsewhere have been able to stop treating as their honeybees have learnt to detect mite infested cells and remove the pupa to prevent mite-reproduction, which leads to decreased mite fertility and population size. However, the majority (72–79%) of UK beekeepers are still treating and it will be many years before most beekeepers in the UK and elsewhere can stop treating for Varroa. In the USA a recent survey found only around 63 (3%) of 2275 respondents stated no advantages to Varroa management and 92% of this group did not treat for Varroa, a very different situation than found currently in the UK. Most beekeepers in the Northern hemisphere have long wished for a silver bullet for the Varroa problem, however, it turns out that the bullet is likely their own bees. Beekeepers need to give their bees time to develop mite resistance as has been done so successfully elsewhere in the world.
Feral Bees
Wild bees are an important issue within the framework of varroa resistance of honeybees. While colonies kept by beekeepers have a limited chance to evolve varroa resistance because they are systematically treated against the mite, this is not the case for wild colonies, which can be a reservoir for naturally selecting varroa resistance genes. Since 1978, before the arrival of the varroa mite, Tom Seeley studied a unique honeybee population of feral colonies nesting in trees in the Arnot Forest, south of Ithaca, NY, USA. In 2002, 15 years after the arrival of varroa, he observed the survival of the colonies. Inspection of the colonies showed that the population as a whole remained stable over three years despite mite infestation and a comparison with susceptible control colonies did not show differences in mite infestation growth rate.
It’s been hypothesized that the persistence of the feral colonies could be due in part to their habit of nesting in small cavities and found that the smaller nest cavities and more frequent swarming of feral colonies contributed to their persistence without mite treatments. These results were confirmed by Seeley (2017), studying the life-history traits of the feral honeybees and of feral colonies in small hives, and comparing them with the life-history traits of the bees before the arrival of the mite. He found that young one-year-old colonies survived less well compared to already established colonies. Moreover, established colonies had a mean lifespan of 5–6 years and a queen turnover (swarming) each summer. Using a population model, he demonstrated that these life-history traits can produce a stable population of colonies. Interestingly, the feral colonies in the 1970s and the 2010s have essentially identical sets of life-history traits before and after the arrival of varroa, which suggests that the feral colonies possess defenses against the mite that are not costly. Because feral colonies in the 2010s have to invest in defenses against the mite, Seeley suggests that small colony size and frequent swarming endow them with good defenses against varroa, so they did not have to evolve costly new defenses against the mites. However, he does not exclude the possibility that the feral colonies have needed to evolve some new defenses against V. destructor, including hygienic behavior and grooming behavior, but that these new defenses should not be costly.
Next Steps
The parasitic mite Varroa destructor spread throughout Europe in the second half of the last century, and now represents the greatest problem for the Western honeybee, Apis mellifera. Since then, the beekeeping industry and hobby beekeepers have had to face a major challenge. The regular use of chemical treatments to control the mite has several disadvantages such as high costs and labor, residues in bee products, and the rapid emergence of mite populations resistant to acaricides. Consequently, there is an urgent need to use alternative control methods for the mite. Natural bee-driven resistance to Varroa is a sustainable, long-term solution, prevents the constant usage of acaricides, will not weaken bees to any other maladies should they arise and may provide an example of parallel evolution with the same three traits arising in populations in several different continents. Also, using mite-resistant honeybees is generally agreed to be the most sustainable way to proceed. Research into varroa resistance in honeybees started in the 1980s and continues to receive a large amount of scientific interest and practical attention in Europe and worldwide. There are several different approaches to obtaining varroa resistant bees.
One approach is to consider that some honeybee populations living with the mite for many generations without varroa control will naturally develop resistance that will contribute to an equilibrium between the parasite and its host. This has been demonstrated with the original host of Varroa spp., the Eastern honeybee Apis cerana, and with A. m. scutellata in southern Africa or imported to South America as the Africanized bee, which has a large degree of resistance to the mite. In Europe and the USA, a few small populations of European strains have been found to be naturally resistant to the mite. The original host, A. cerana, has co-evolved with its host over millions of years, which is not the case in A. mellifera. However, the presence and survival of these populations demonstrates that a period of less than 100 years can be enough to build up an equilibrium between the host and the parasite.
While naturally resistant strains mainly consist of feral colonies with no impact of the beekeeper, another approach can be mass selection using a large group of varroa infested honeybee colonies, which are allowed to live with the mite without any treatment and either die or survive. This has been called the “Bond test” (“Live and let die!”) and was developed in Sweden and in France and then in a few other European countries. Another label for this might be Darwinian Beekeeping.
Finally, a more academic approach for artificial selection is to develop a genetic selection based on chosen phenotypic characters and quantitative genetic tools. This approach has been utilized more or less successfully since the 1980s by several different research teams around the world. We can see this outlined in the process currently used by a small group in the UK and called the Westerham Beekeepers program (images below from their slide show. Consider picking up the book: The Honey Bee Solution to Varroa. A practical guide for beekeepers). This is how it works…you may want to try this in your own apiary.

Phase 1: Begin using one of the biotechnical methods below to transition off treatments (listed in rank of commercial profitability). Each of these methods promise to efficiently reduce mite loads, taking the problem away from the bees and in effect, replicate the role of treatments. They cause a brood break that is also found with artificial swarming techniques.
- Total Brood Removal (TBR) – July brood removal and July OA treatment
- Royal Cell Insertion (CI) – July old queen replacement and August OA treatment
- Queen caging (QC) – Cage queen in July and August OA treatment
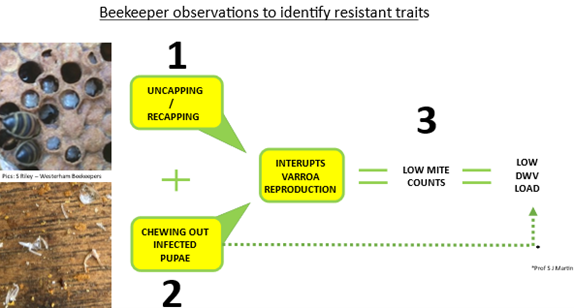
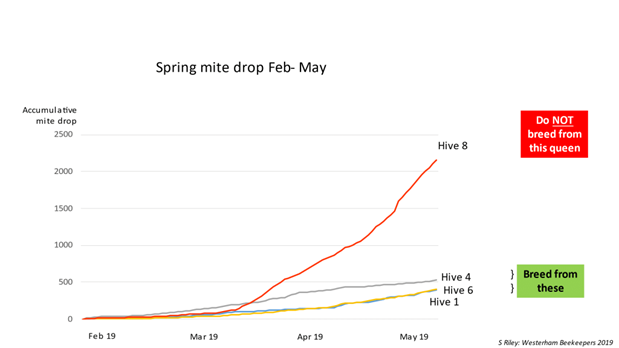
Phase 2: Monitor to see which colonies have natural resistant traits – treatments keep weak traits circulating which is the opposite of good husbandry. Here are the traits to look for.
- Observing uncapping/recapping during colony inspections – good hygienic behavior.
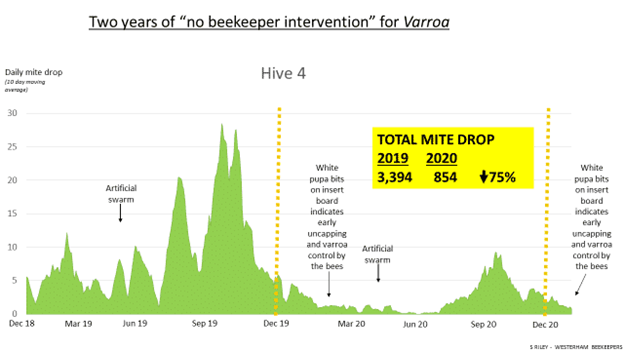
- Monitor for pupae exoskeletons on the bottom – Interruption of Varroa reproduction.
- Low accumulative mite drop numbers (over 3 months) & Deformed Wing Virus (DWV)


Reference Materials
- Steve Riley (Westerham Beekeepers)
- Varroa Resistant Honeybees Website
- Resistance to Varroa destructoris a trait mainly transmitted by the queen and not via worker learning
- Rapid parallel evolution overcomes global honey bee parasite
- Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations
- Spatial distribution of recapping behaviour indicates clustering around Varroa infested cells
- Elevated recapping behaviour and reduced Varroa destructor reproduction in natural Varroa resistant Apis mellifera honey bees from the UK
- Deformed wing virus prevalence and load in honeybees in South Africa
- Parallel evolution of Varroa resistance in honey bees: a common mechanism across continents?
- Global Evidence for Varroa Mite-Tolerant Bees with Dr. Stephen Martin (YouTube Video)
- Varroa resistant honey bee populations – Dr. Melissa Oddie (YouTube Video)
- Recapping and mite removal behaviour in Cuba: home to the world’s largest population of Varroa resistant European honeybees
- A survey of UK beekeepers’ Varroa treatment habits
- Varroa resistance in Apis cerana: a review
- Chemical detection triggers honey bee defense against a destructive parasitic threat
- Pupal cannibalism by worker honey bees contributes to the spread of deformed wing virus
- Geographical distribution and selection of European Honeybees Resistant of Varroa
- Ancient, veteran and other listed trees as nest sites for wild-living honey bee, Apis mellifera, colonies
- Varroa Control Past and Future
- Natural Beekeeping Trust
- Sustainable Beekeepers Guild of Michigan
- Total Brood Removal and Other Biotechniques for the Sustainable Control of Varroa Mites in Honey Bee Colonies: Economic Impact in Beekeeping Farm Case Studies in Northwestern Italy
- Biotechnical Control of Varroa in Honey Bee Colonies: A Trade-Off between Sustainable Beekeeping and Profitability?
